
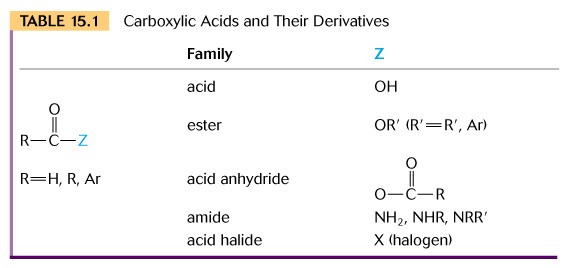
I. CARBOXYLIC ACIDS
(1) Introduction - Carboxylic acids contain one or
more carboxyl functional groups (-COOH). The IUPAC nomenclature drops
the ( -e ) of the suffix of the Alkane name and adds ( -oic acid).
Review the names of the first ten Alkanes and convert these names to the corresponding carboxylic acid.
The carboxyl functional group is polar, both at the carbonyl part and at the hydroxyl part because of the difference in electronegativity value of carbon and oxygen, and oxygen and hydrogen. Thus carboxylic acids will hydrogen bond with themselves and any other appropriate polar molecule, and this structure will influence their physical properties and their chemical properties as we have seen in other cases.
Note that if the number of total carbons is more than a one, for example six, then five of the carbons are hydrocarbons and non-polar.
The resulting molecule then has a hydrophilic part (the carboxyl group) and a hydrophobic part (the other five carbons with their associated hydrogen atoms).
- One area will be soluble in water and the other not soluble in water.
- Note also that the hydrocarbon part has much reduced carbon.
- The carboxyl group differs from a hydroxyl group in having a delocalized negative charge and this results in resonance stability.
- Note this means that either oxygen (carbonyl or hydroxyl) can double bond when proton is removed from hydroxyl.
(2) Chemical Reactions of Carboxylic Acids -
(a) Acidity - carboxylic acids ionize in water to produce a proton and are thus classified as acids. The extent of ionization is low, in the range of 1-2%, and we would term them as weak acids. Recall the earlier discussion of acids, bases, and buffers, plus the titration curve of ethanoic acid (acetic acid). The pK of this acid is 4.76 x 10-5 M., and was a good buffer in the range of plus & minus one pH unit around the pK value. In addition, recall the concept of the conjugate base of weak acids, and note that the carboxylate ion is a base. The carboxylate ion (COO -) is an anion of weak acids and are themselves relatively good bases, especially toward strong proton donors.
(b) Conversion to Esters - carboxylic acids can be reacted with alcohols to form esters by removing the elements of water i.e. hydrogen from one and hydroxyl from the other. Methanoic acid plus methanol gives methyl methanoate. Write out this reaction.
(c) Nucleophilic Substitution - the nucleophilic acyl substitution reaction of the carboxyl group is different from that of aldehydes/ketones since we now have a group capable of stabilizing a negative charge (oxygen) and leaving the reacting molecule with this charge. Thus the nucleophilic addition step is followed by a leaving group and we term the net reaction as a substitution. Note that the term acyl group means a carbonyl group with an unspecified R- group plus an unshared electron ready for bonding, i.e. a carboxylic acid minus it's hydroxyl group.
(d) Conversion to Acid Anhydrides - the reaction between two acid molecules gives rise to the acid anhydride plus water. Note this links two carbonyl functional groups by an oxygen atom or bridge.
(e) Conversion to Amides - the reaction between a carboxylic acid and ammonia produces an amide plus water, i.e. the hydroxyl group is replaced by an amino group. The textbook covers amides in the amine chapter, but it is useful to make note of it here with the carboxylic acids.
(f) Fatty Acids - are even numbered long-chained monocarboxylic acids, and are very important as energy storage molecules. They may be saturated or unsaturated, i.e. contain one or more carbon-to-carbon double bonds, and their high-energy content is related to their hydrocarbon structure. When we get to the lipid chapter, we will study their structure as part of triacylglycerols and phospholipids.
II. ESTERS
(1) Introduction - Esters, as noted above are formed
from a carboxylic acid and an alcohol, where the alcohol replaces the
hydroxyl group on the carboxyl group. The IUPAC name is obtained by dropping
the (-ic acid) and replacing it with (-ate). For example, the reaction
of ethanoic acid and methanol gives water plus methyl ethanoate. The "ester
bond" between the carbonyl carbon and oxygen is easily broken and provides
a major point of the various reactions of esters. Low molecular weight
esters have pleasant odors.
(2) Chemical Reactions - The chemical reactions of esters are numerous and include:
(a) Hydrolysis reactions produce the corresponding acid and alcohol which formed the ester.
(b) Ammonolysis reactions of an ester involve the addition of ammonia to the ester, under the proper conditions, and the product is an amide.
(c) Alcohololysis reactions of an ester involve the addition of an alcohol, with an acid catalytic agent, results in the interchange of the alkyl groups on the carboxyl oxygen.
(d) Condensation reactions of two esters results in the synthesis of a beta-keto-ester. This reaction occurs in two steps, the first one being the conversion of one ester to a nucleophilic donor via loss of an alpha-hydrogen with a base catalyst, and the second step uses this donor to carryout a nucleophilic addition to the carbonyl carbon of the second ester. Such condensation reactions of esters are very important in the metabolism of cells, and this is a good place to be sure that you understand the basic concept of these reactions.
(e) Thiol Esters are used by cells, rather than the common reactive organic acid halogens or anhydrides, as important reagents. The pKa value of a typical alkane thiol is about 10, and this value is about halfway in acid strength between carboxylic acids, whose value is about 5, and alcohols whose value is 16. Thus the thiolate anions (RS-) can act as leaving groups in nucleophilic acyl substitution reactions. A major molecule here is coenzyme A which contains the amino acid cysteine, whose R-group is (-CH2SH). Coenzyme A works with the various enzymes of energy metabolism to accept and then to donate acyl groups.
III. ACID ANHYDRIDES
(1) Introduction - The combination of two acids
via a dehydration reaction, i.e. the removal of a molecule of water, produces
an acid anhydride. The IUPAC naming drops the word acid and adds anhydride.
The linkage (-CO-O-CO-) defines this family.
(2) Chemical reactions - The Acid Anhydrides
are very reactive and follow a similar pattern to that of esters as we
have already seen. They need to be protected from water (moisture) since
they readily undergo hydrolysis.
(3) Organophosphate Esters & Anhydrides -
In cells the most widely distributed kinds of esters and anhydrides are
anions of esters of phosphoric acid, diphosphoric acid, and triphosphoric
acid. Recall that phosphoric acid (H3PO4) has three
hydroxyl groups and phosphorus doubled bonded to oxygen, thus it readily
forms esters and anhydrides as the phosphate. Each hydroxyl has a specific
pK value, and the second one is near pH 7. The energy metabolism of all
cells is based on the two molecules ATP and ADP, and we will return
to these molecules during the metabolism section. In addition, the
structure of nucleic acids (DNA & RNA) also utilizes the unique properties
of phosphoric acid to link together sugars, which provide the framework
for the addition of the purine and pyrimidine bases. We will cover
molecular genetics late in the course.